
A three-year-old boy has astounded doctors with his progress after becoming the first person in the world with his devastating disease to receive a ground-breaking gene therapy.
Oliver Chu has a rare, inherited condition called Hunter syndrome – or MPSII – which causes progressive damage to the body and brain.
In the most severe cases, patients with the disease usually die before the age of 20. The effects are sometimes described as a type of childhood dementia.
Due to a faulty gene, before the treatment Oliver was unable to produce an enzyme crucial for keeping cells healthy.
In a world first, medical staff in Manchester have tried to halt the disease by altering Oliver’s cells using gene therapy.
Prof Simon Jones, who is co-leading the trial tells the BBC: “I’ve been waiting 20 years to see a boy like Ollie doing as well as he is, and it’s just so exciting.”
At the centre of this remarkable story is Oliver – the first of five boys around the world to receive the treatment – and the Chu family, from California, who have put their faith in the medical team at Royal Manchester Children’s Hospital.
A year after starting the treatment, Oliver now appears to be developing normally.
“Every time we talk about it I want to cry because it’s just so amazing,” says his mother Jingru.
The BBC has followed Oliver’s story for more than a year – including how scientists in the UK first developed the pioneering gene therapy and how the medical trial they are conducting almost didn’t get off the ground due to lack of funds.
Stem cell removal – December 2024
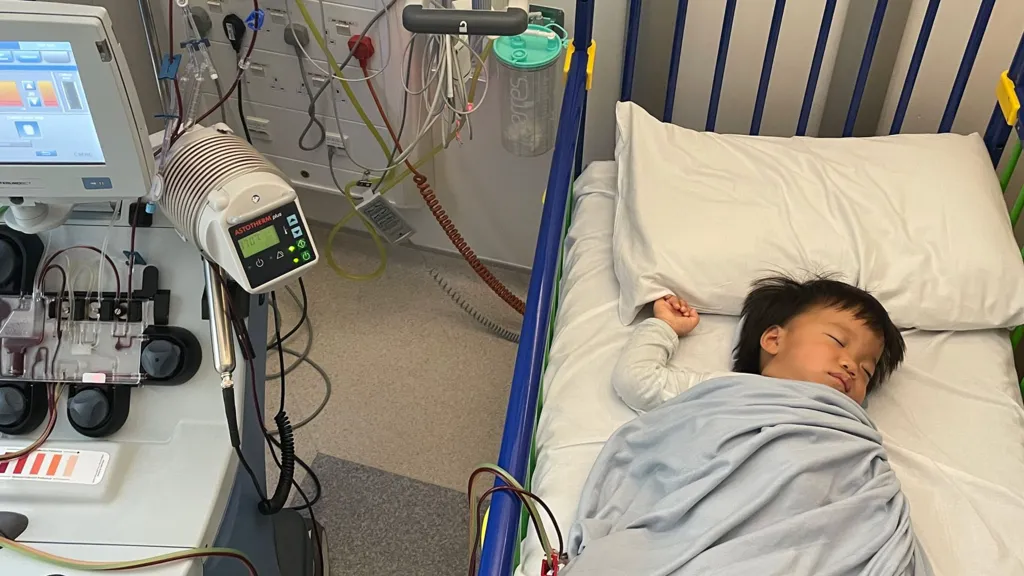
We first meet Oliver and his dad Ricky in December 2024 at the clinical research facility at Royal Manchester Children’s Hospital. It’s a big day.
Since being diagnosed with Hunter syndrome in April, Oliver’s life – like that of his elder brother, Skyler, who also has the condition – has been dominated by hospital visits.
Skyler had shown some late development in speech and coordination, but this had initially been put down to being born during Covid.
Ricky tells me his sons’ diagnosis came as a complete shock.
“When you find out about Hunter syndrome, the first thing the doctor tells you is ‘Don’t go on the internet and look it up because you’ll find the worst cases and you’ll be very, very disheartened’.”
“But, like anybody, you look it up and you’re like, ‘Oh my goodness, is this what’s going to happen to both my sons?’”
Children are born apparently healthy, but around the age of two they start to show symptoms of the disease.
These vary and can include changes to physical features, stiffness of the limbs and short stature. It can cause damage throughout the body, including to the heart, liver, bones and joints and in the most serious cases can lead to severe mental impairment and progressive neurological decline.
Hunter syndrome almost always occurs in boys. It’s extremely rare, affecting one in 100,000 male births in the world.
Until now, the only medicine available for Hunter syndrome was Elaprase, which costs around £300,000 per patient, per year and can slow the physical effects of the disease. The drug is unable to cross the blood-brain barrier and so does not help with cognitive symptoms.
But today, Oliver is being hooked up to a machine and having some of his cells removed – the first crucial step in trying to halt his genetic disorder in this one-off treatment.
“His blood is being passed through a fancy machine that is collecting a specific type of cell called stem cells which will be sent to a lab to be modified and then given back to him,” Dr Claire Horgan, consultant paediatric haematologist explains.
Oliver’s cells are tweaked
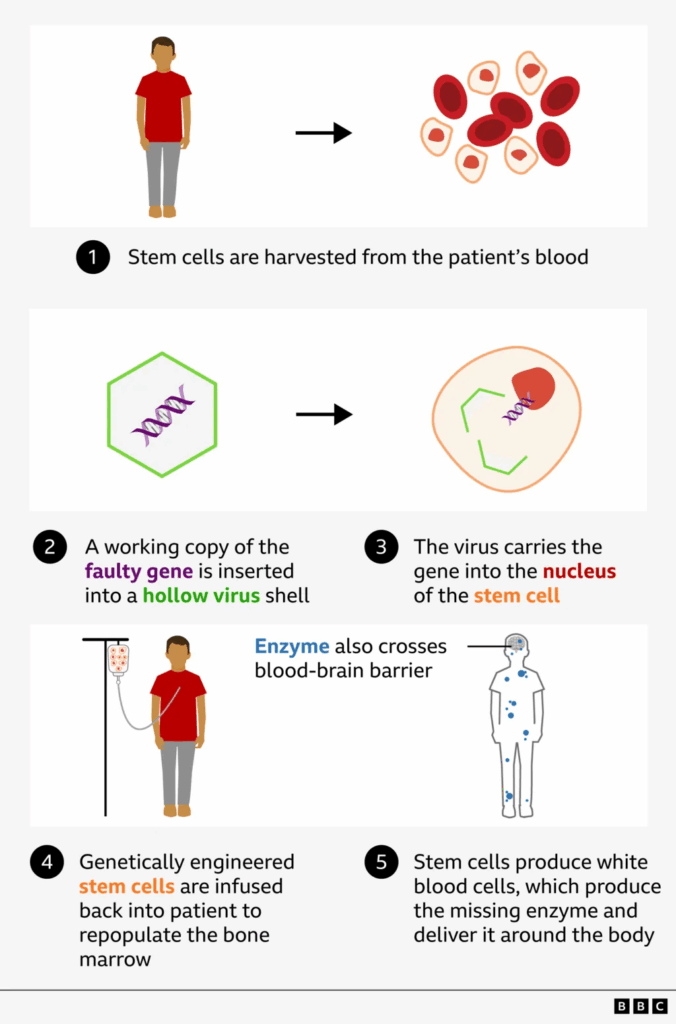
Oliver’s cells are carefully packaged and sent to a laboratory at Great Ormond Street Hospital (GOSH) in London.
In Hunter syndrome, a genetic error means that cells are missing the instructions for making an enzyme, iduronate-2-sulfatase (IDS), essential for breaking down large sugar molecules which over time accumulate in tissues and organs.
Scientists insert the missing IDS gene into a virus, which has its genetic material removed so that it can’t cause disease.
A similar method has been used in other gene therapies, such as the treatment for another rare inherited condition, MLD.
Dr Karen Buckland, from the Cell and Gene Therapy Service at GOSH, explains: “We use the machinery from the virus to insert a working copy of the faulty gene into each of the stem cells.
“When those go back to Oliver, they should repopulate his bone marrow and start to produce new white blood cells and each of these will hopefully start to produce the missing protein [enzyme] in his body.”
There still remains the issue of how to get enough of the missing enzyme into the brain.
To overcome this, the inserted gene is modified so that the enzyme it produces crosses the blood-brain barrier more efficiently.
Infusion day – February 2025
We next meet Oliver back at the clinical research facility at Royal Manchester Children’s Hospital.
This time he’s with his mum Jingru, while Ricky has stayed in California to look after Skyler.
There is a sense of anticipation as a member of the research team opens a large metal cryopreservation tank where Oliver’s gene edited stem cells are frozen, having been transported back from GOSH.
A small, clear infusion bag is removed and slowly brought to body temperature in a tray of liquid.
After multiple checks, a nurse draws the clear fluid containing around 125 million gene-modified stem cells, into a syringe.
Oliver is used to hospitals, but is fretful, and wriggles as the research nurse slowly injects the treatment, about a cup full, into a catheter in his chest.
Jingru holds Oliver steady in her arms. After 10 minutes, the infusion is done.
An hour later, a second, identical infusion is made. Oliver continues to watch cartoons on a portable screen, oblivious to the potential importance of what’s just happened.
And that’s it. The gene therapy is complete. It seems to be all over rather quickly, yet the ambition here is huge: to stop Oliver’s progressive disease in its tracks, in a one-off treatment.
After a couple of days, Oliver and Jingru fly back to California. Now the family, and the medical team must wait to see if the gene therapy has worked.
Early signs of progress – May 2025

In May, Oliver is back in Manchester for crucial tests to see if the gene therapy is working. This time the whole family is here.
We meet in a park in central Manchester and it’s immediately clear that things are looking good.
Oliver is more mobile and inquisitive than I’ve seen him. Admittedly, he now has the freedom to play and is out of hospital, but he appears brighter and healthier.
Ricky is thrilled: “He’s doing really well. We have seen him progressing in his speech, and mobility. In just three months he has matured.”
The really big news is that Oliver has been able to come off the weekly infusion of the missing enzyme.
“I want to pinch myself every time I tell people that Oliver is making his own enzymes,” says Jingru. “Every time we talk about it I want to cry because it’s just so amazing.”
She tells me he is “so different” from before the treatment, is talking “a ton” and is engaging more with other children.
It is lovely to finally meet five-year-old Skyler who is very protective and caring towards his younger brother.
“My wish upon the star is for Skyler, to be able to get the same treatment,” says Ricky. “It feels like Oliver has got a reset in his life, and I want the same thing for Skyler, even though he’s a bit older.”
Initially it was thought that Oliver was too old for the trial, as the treatment cannot reverse existing damage, but tests showed he was still largely unaffected.
Skyler seems to take delight in the world around him, and is keen to hold my hand and chat as we walk to the park.
Ricky explains that Skyler has delayed development in speech and motor skills, but is undergoing infusion therapy, which gets the treatment to his body, but not his brain.
‘Eternally grateful’
Oliver returns to Manchester every three months for a few days of follow-up tests.
In late August, further checks confirm the gene therapy is working.
Oliver is clearly thriving, and to date is now nine months post treatment.
Prof Jones, whom Oliver calls Santa because of his white beard, is beaming: “Before the transplant Ollie didn’t make any enzyme at all and now he’s making hundreds of times the normal amount.
“But more importantly, we can see he’s improving, he’s learning, he’s got new words and new skills and he’s moving around much more easily.”
However, Prof Jones exercises a degree of caution: “We need to be careful and not get carried away in the excitement of all this, but things are as good as they could be at this point in time.”
On the rooftop garden at the hospital, Oliver plays with his dad.
“He’s like a completely different child. He’s running around everywhere, he won’t stop talking,” says Ricky.
“The future for Ollie seems very bright and hopefully this means more kids will get the treatment.”
In all, five boys have been signed up for the trial, from the US, Europe and Australia. None are from the UK as patients here were diagnosed too late to qualify.
All the boys will be monitored for at least two years. If the trial is deemed a success, the hospital and university hope to partner with another biotech firm in order to get the treatment licensed.
Prof Jones says the same gene therapy approach is being applied to other gene disorders.
There are similar treatments on trial in Manchester for MPS type 1 or Hurler syndrome and MPS type 3 or Sanfilippo syndrome.
Ricky and Jingru say they are “eternally grateful” to the Manchester team for allowing Oliver to join the trial.
They say they are astonished by his progress in recent months.
Oliver’s now producing the missing enzyme and his body and brain are healthy.
“I don’t want to jinx it, but I feel like it’s gone very, very well,” says Ricky.
“His life is no longer dominated by needles and hospital visits. His speech, agility and cognitive development have all got dramatically better.
“It’s not just a slow, gradual curve as he gets older, it has shot up exponentially since the transplant.”
The trial that almost never was

Researchers at the University of Manchester led by Prof Brian Bigger had spent more than 15 years working on creating the gene therapy for Hunter syndrome.
In 2020 the university announced a partnership with a small US biotech company Avrobio, to conduct a clinical trial.
But three years later the company handed back the licence to the university, following poor results from another gene therapy study and a lack of funds.
The first-in-human trial, which would soon help Oliver, was in jeopardy before it had even begun.
Prof Jones: “We had to move very quickly to try to save the whole idea and find another sponsor and another source of funding.”
It was then that British medical research charity, LifeArc, stepped in, providing £2.5m of funding.
CEO Dr Sam Barrell said: “A huge challenge for the more than 3.5 million people in the UK living with rare conditions, is getting access to effective treatments – currently 95% of conditions have none. “
The Chu family are relieved the trial didn’t come to a halt and now hope Skyler may one day benefit from the same gene therapy as his brother.
“I would walk to the end of the earth, backwards, forwards, upside down, barefoot, to make sure my kids have a better future,” says Ricky.




